Reuse of leftover allograft from previous surgery: A cost-saving opportunity or a hidden threat?
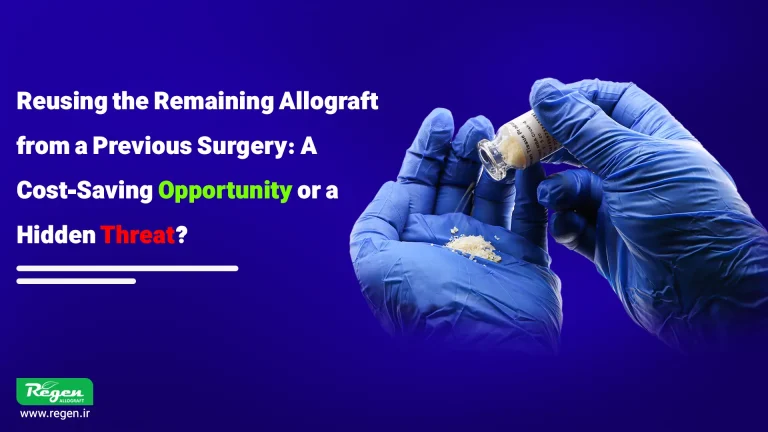
Table of contents
At first glance, reusing allograft from a previous surgery may seem like a logical and cost-effective measure, especially when these materials are expensive and account for a significant portion of the treatment budget. However, the key question is whether such a practice is scientifically and clinically safe?
From the moment the sterile packaging of the graft material is opened, its surface is directly exposed to the surgical environment and airborne particles. Even when all aseptic principles are strictly followed, microorganisms can still be transferred from instruments, gloves, or the surgical field to the graft material. These microscopic contaminations may initially show no signs, but upon reuse, they can lead to infection, inflammation, and even complete failure of the implant treatment.
In addition to microbial risks, changes in the physical and biological properties of the graft material after the package is opened are also a significant concern. Exposure to moisture, air, and temperature fluctuations can alter the material’s structure, reducing its resorption capacity or ability to integrate with the host bone.
Therefore, although reusing graft material may appear to be an economical measure on the surface, in practice, it poses a hidden threat to patient safety and treatment success.
International standards in the field of bone grafting and reconstruction explicitly emphasize that each graft material should be used for only one patient and during the same surgical session, with any remaining material disposed of in accordance with safety protocols.
In conclusion, it can be said that true economy in surgery lies not in reusing materials, but in preventing complications, reducing contamination risks, and safeguarding patient health.
Of course. Here is the translation, maintaining the same tone and context:
After surgery is completed, some of the biomaterial may remain in its packaging. The desire to use this valuable biomaterial for another patient, or even for the same patient in a different session, is understandable. Reducing waste and conserving resources are natural concerns for any healthcare facility.
But is this practice scientifically and legally justifiable? The short answer is no. Even when the packaging appears intact, from the moment it is opened, the graft material is exposed to the environment, air, and surgical instruments. This exposure can lead to microbial contamination, jeopardizing patient safety.
Therefore, allografts and many transplant biomaterials are manufactured as Single-Use items. This limitation exists not only to maintain sterility and prevent infection but also to guarantee the preservation of the graft material’s physical and biological properties. Any attempt at reuse can alter the material’s structure and disrupt its integration process with the host bone, which may ultimately lead to treatment failure.
Therefore, short-term savings from reusing biomaterials cannot replace patient safety and clinical success. Adhering to manufacturer guidelines and scientific standards remains the primary and most reliable way to ensure optimal treatment outcomes.

Definition (Single Use)
Single Useor refers to products that are designed exclusively for one patient and one surgical procedure. Once the packaging is opened, their sterility can no longer be guaranteed, and their reuse is prohibited both scientifically and legally.
According to the regulatory requirements of the U.S. and European authorities, allografts must strictly be single-use, and any form of reuse is considered a direct violation of these standards. The packaging of these products typically includes standard phrases or symbols, such as:
- Do Not Re-Use
- Medical Device Is Intended for Single Use Only
- Use Only Once
These symbols are defined according to the ISO 15223-1 standard (Medical Device Symbols) and their purpose is to protect the patient and ensure treatment safety. Any disregard for these guidelines can lead to infection, treatment failure, or legal issues for the healthcare facility.
Risks of Microbial Contamination and the Importance of Not Reusing Graft Materials
Why is reusing graft materials incorrect and dangerous? The answer lies in breaking the chain of sterility.
Correct. After the graft material is placed in the surgical environment and the package is opened, the product loses its factory sterility, and its surface is exposed to the air and the environment of the operating room. This environment, even under controlled conditions, is filled with microorganisms and contaminated airborne particles that can adhere to the surface of the graft material.
Indeed, contaminants that settle on the material’s surface cannot be eliminated by any conventional sterilization method. Reusing these materials directly increases the risk of infection, inflammation, and treatment failure.
Emphasizing non-reuse is not merely a commercial recommendation from companies; rather, it is a gold standard in science and clinical practice aimed at:
- Patient Safety
- Guaranteeing treatment success
It is established. Adhering to this standard is one of the most important principles in surgical and tissue regeneration protocols, ensuring that every patient is treated in a completely sterile environment with maximum safety.
Frequently Asked Questions (FAQ)
Can’t the remaining graft be used for the same patient in the next surgical session?
Answer: No. The risk of microbial contamination exists from the moment the package is opened until any subsequent session.
Storing it even under ideal conditions cannot guarantee sterility and may lead to infection in the surgical site.
Can we store the remainder in the refrigerator under sterile conditions?
Answer: No, the refrigerator is a humid environment and does not sterilize its contents; it only slows the growth of some microbes.
The risk of contamination upon reopening and using the product still persists.
Doesn’t this cause a waste of money?
Answer: No, the cost of treating a severe infection, graft failure, or potential legal complications is far greater than the cost of discarding a small amount of graft material.
Patient safety is not a cost; it is a non-negotiable investment.
Practical Guidelines for Surgeons in Using Allografts
To preserve patient safety and ensure treatment success, adhering to the following practical guidelines in the use of graft materials is very important:
1. Packaging Inspection:
Always check the package integrity before use. Any tear, hole, or damage to the packaging renders the product unusable, and using it can increase the risk of infection.
2. Sterile Opening:
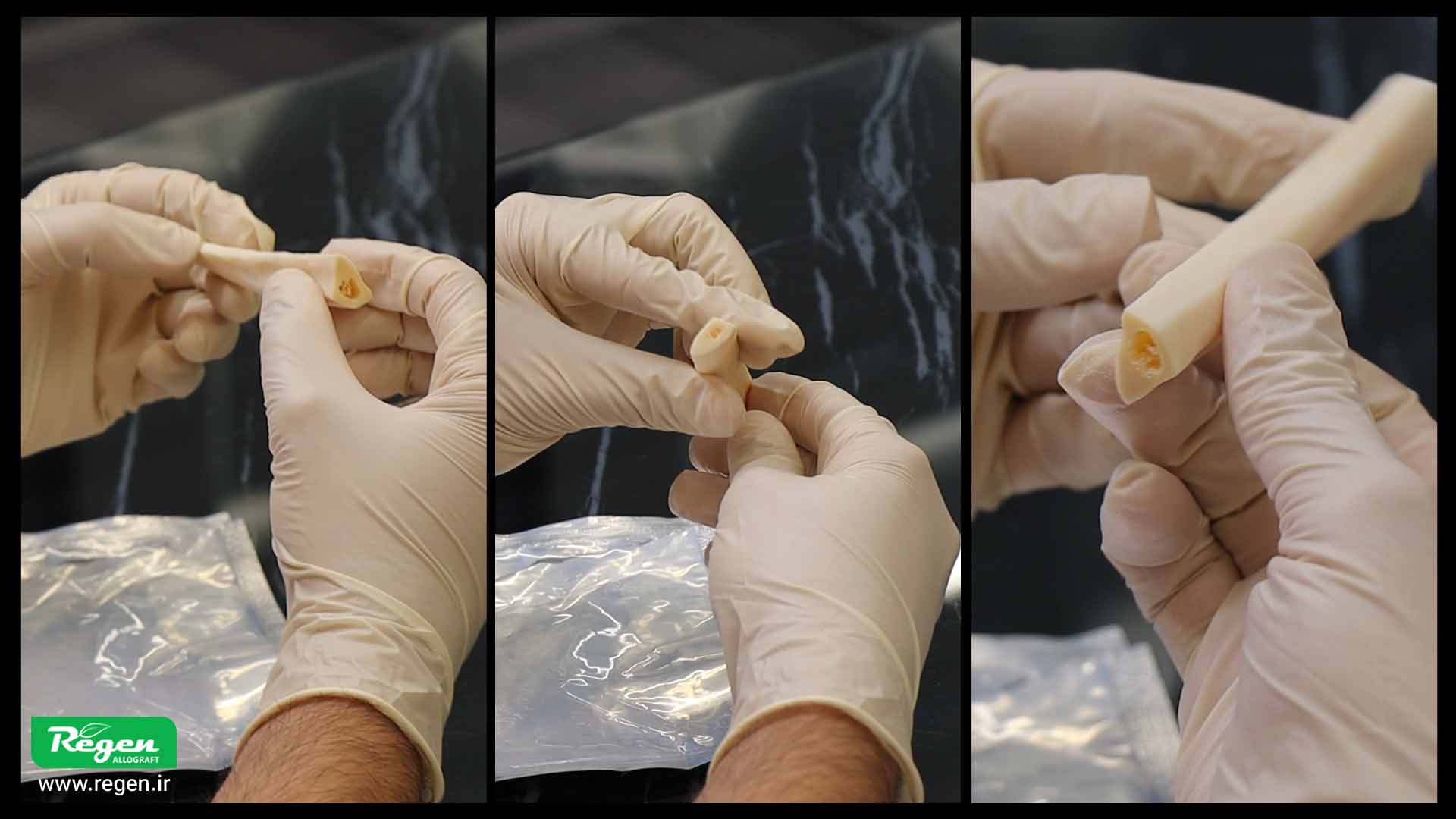
Open the package only at the time of use and in a completely sterile environment. Additionally, minimize the time between opening the package and applying the material. To reduce the possibility of contamination, during the product rehydration process, you can place sterile aluminum foil over the product container.
3. Immediate Use:
Use the allograft immediately after preparation. Never store the hydrated material for later use, as over time, the likelihood of contamination and reduction in the biological properties of the material increases.
4. Documentation:
Record the lot number, expiration date, and time of product use in the patient’s record. This action is not only essential for tracking product quality but also holds significant importance in legal matters and clinical standards.
By adhering to these guidelines, surgeons can ensure patient safety and maximize the chances of treatment success.
Patient safety is not a cost;
it is a non-negotiable investment.
Practical Guidelines for Surgeons in Using Allografts
1. Packaging
Before use, check the package integrity. Any tear, hole, or damage renders the product unusable and can increase the risk of infection.
2. Sterile Opening
Open the package only at the time of use and in a completely sterile environment. The time between opening the package and use should be minimal. To reduce contamination, during hydration, sterile foil can be placed over the container.
3. Immediate Use
Use the allograft immediately after preparation. Never store the hydrated material for future uses; as time passes, the probability of contamination and decrease in biological quality increases.
4. Documentation
Record the lot number, expiration date, and time of product use. This is essential for tracking quality, compliance with standards, and legal matters.
Summary of the Discussion
The Single-Use principle regarding allografts is not merely a commercial recommendation, but a scientific and ethical standard established to protect patients and surgeons. For optimal use and waste reduction, the required volume of graft material should be estimated prior to surgery using CBCT imaging. Precise selection of the appropriate volume and size of graft material based on each case’s needs not only enhances treatment efficacy but also aids in optimal cost management. Ultimately, it must be noted that the costs of complications arising from infection or treatment failure are far greater than the price of the allograft, and adherence to single-use standards remains the primary priority in tissue reconstruction and implant surgery.
How useful was this post?
Click on a star to rate it!
Average rating 5 / 5. Vote count: 1
No votes so far! Be the first to rate this post.
Relevant Posts

Free consultation
Do you need counseling?
The professional and specialized team at Allograft is ready to assist you