Soft Derm
Choose File
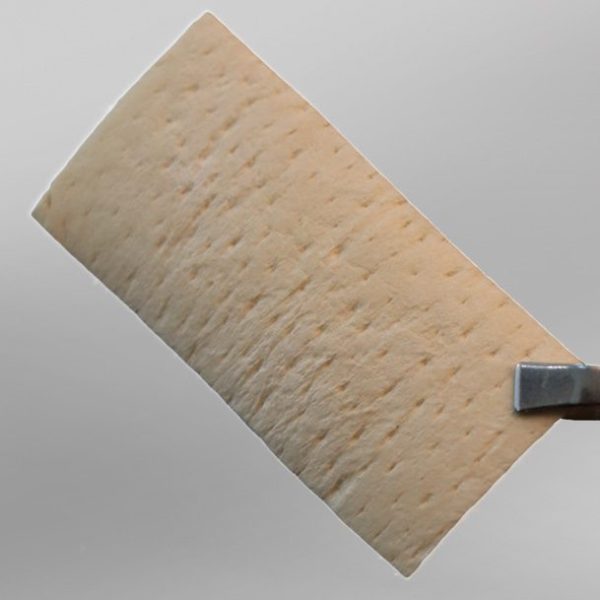
The Acellular Dermis Membrane (ADM) is an allograft derived from human dermal tissue, in which the epithelial layer has been removed while maintaining the integrity of the basement membrane. This preserved barrier prevents the penetration of microorganisms, thereby allowing the product to remain exposed in the oral cavity.
On the opposite side, the dermal side of the product contains a collagenous matrix composed of type I and type III collagen fibers, which provide structural strength. In addition, the preserved reticular and elastin fibers in the Acellular Dermis Membrane ensure flexibility and confer its unique properties.
ADM serves as a scaffold for host cells, ultimately promoting vascularization within the matrix and supporting tissue regeneration.
Beyond its application as a barrier membrane in bone regeneration procedures, ADM—depending on its thickness—also acts as an excellent substitute for soft tissue augmentation, particularly in cases of contour
Product Description
Benefits
- Biocompatibility and no need for hypersensitivity reaction test before usage.
- All human sources are safe and traceable for a long period of time.
- We also screen all donors for serological tests such as ELISA and PCR assays with microbiological culture tests and assuring of tissue safety before processing.
- Good volume enhancement is one of ITP Acellular Dermis characteristics.
- It helps to maintain normal tissue structure and aesthetic reconstruction.
Clinical Application
- Soft tissue augmentation and volume enhancement.
- GTR, GBR, dural defect repairment, herniation repair, mamoplasty, contracture reduction in scar treatment, tympanoplasty, septoplasty, etc …
Storage Condition
Store this product at room temperature (15 to 30ºC) for membranous form and 1 to 10ºC for Collagel (Refrigeration is needed.) Avoid excessive heat & humidity. Membranes have 5 year shelf life.
Contact us
Product Code | Product Name | Therapeutic Area |
|---|---|---|
5010014 | Lyophilized Acellular Dermis Membrane T:1-1.8 mm S:15×20 mm | Dental |
5010015 | Lyophilized Acellular Dermis Membrane T:1-1.8 mm S:20×30 mm | Dental, ENT |
5010016 | Lyophilized Acellular Dermis Membrane T:1-1.8 mm S:20×40 mm | Dental, ENT |
5010049 | Lyophilized Acellular Dermis Membrane T:0.3- 0.4 mm S:10×10 mm | Content |
5010050 | Lyophilized Acellular Dermis Membrane T:0.4-0.6 mm S:10×10 mm | Content |
5010051 | Lyophilized Acellular Dermis Membrane T:0.6-1 mm S:10×10 mm | Content |
5010052 | Lyophilized Acellular Dermis Membrane T:1-1.8 mm S:10×10 mm | Content |
5010017 | Lyophilized Acellular Dermis Membrane Customized Size/Thickness | Content |
Contact us
Get expert advice
Contact our experts for free guidance and advice, or read our About Us page to learn more about us.
Related content
Some of our products
The acellular dermis membrane collagen membrane, contains basement membrane on one of its surfaces. It can provide a dense and impenetrable layer against microorganism and pathogen penetration into deeper layers.
So the membrane surface can stay exposed in high-perfused tissue of the oral cavity. The product origin is the collagen membrane (skin dermal layer), which consists of strong collagen type I and III fibers that strengthen the product, and also elastic fibers causing flexibility. This collagen membrane results in product’s unique features.
Freeze-dried bone allograft as Bone grafting such as Cube, Matchstick and Block forms has revolutionized orthopedic and maxillofacial surgeries, offering distinct advantages due to their unique compositions. Cube and Matchstick configurations consist solely of cancellous bone, while block formations comprise a core of cancellous bone enveloped by a cortical surface. These allograft are meticulously processed to decellularization and ensure sterilization. The resulting grafts provide essential structural support and seamlessly integrate with the recipient’s bone, promoting the formation of new bone.
Cube and block bone allografts effectively address osseous deficiencies caused by various conditions, from post-traumatic injuries to degenerative diseases and reconstructive procedures. In dental implantology, they serve as a reliable scaffold for augmenting deficient mandible and maxilla structures and facilitating successful implant placement. Moreover, in reconstruction surgeries, these grafts significantly contribute to restoring maxillofacial and skeletal, enabling patients to regain both physical form and function.
Ongoing refinement of bone grafting techniques, along with continued research and innovation, will further enhance the applications and outcomes of cube and block bone allografts, elevating the standard of care in orthopedic and maxillofacial surgeries.