A Comprehensive Review of Allograft Sterilization with Gamma Irradiation: Ensuring Safety

Table of contents
Safe Sterilization of Bone Allografts and Collagen Membranes Using Gamma Irradiation
Allograft refers to the transplantation of tissue from one individual to another (non-related donor). Today, various types of bone and skin allografts are widely used as effective alternatives to autografts (the patient’s own tissue) and xenografts (animal-derived tissue) in the fields of orthopedics, dentistry, oral and maxillofacial surgery, and soft tissue reconstruction. These allografts include bone grafts in different forms (such as lyophilized bone powder, bone chips, and bone putty, as well as other matrix-based scaffolds like demineralized bone matrix), collagen membranes (for periodontal and bone regeneration), and collagen-based grafts such as skin grafts, fascia membranes, dermal membranes, and pericardial membranes. All lyophilized Regen Allograft products undergo a final sterilization process in collaboration with the Atomic Energy Organization.
Regen Allograft products serve as biological scaffolds for filling bone defects, volume augmentation, collagen membranes for applications in dentistry and implantology, and biological dressings for wound coverage. Their main advantage over autografts is the elimination of donor-site morbidity and the reduction of pain and complications associated with tissue harvesting. Nevertheless, the biosafety of . these donor-derived tissues remains a critical concern, requiring meticulous processing and final sterilization before clinical use.

The importance of sterilization in tissue transplantation
Terminal sterilization, aiming for a Sterility Assurance Level (SAL) of 10⁻⁶ (meaning a maximum probability of one viable microorganism in one million samples), is essential to ensure the complete safety of transplanted tissues. Without a definitive sterilization step, the risk of infection transmission can never be reduced to zero. Therefore, standard-setting organizations such as the American Association of Tissue Banks (AATB) mandate strict compliance with a reliable terminal sterilization process. This step is particularly critical for bone and skin grafts used in patients with compromised immunity or in the management of large wounds.
All lyophilized products of this company undergo gamma irradiation in their final packaging stage (after primary and secondary packaging) to guarantee complete sterility.
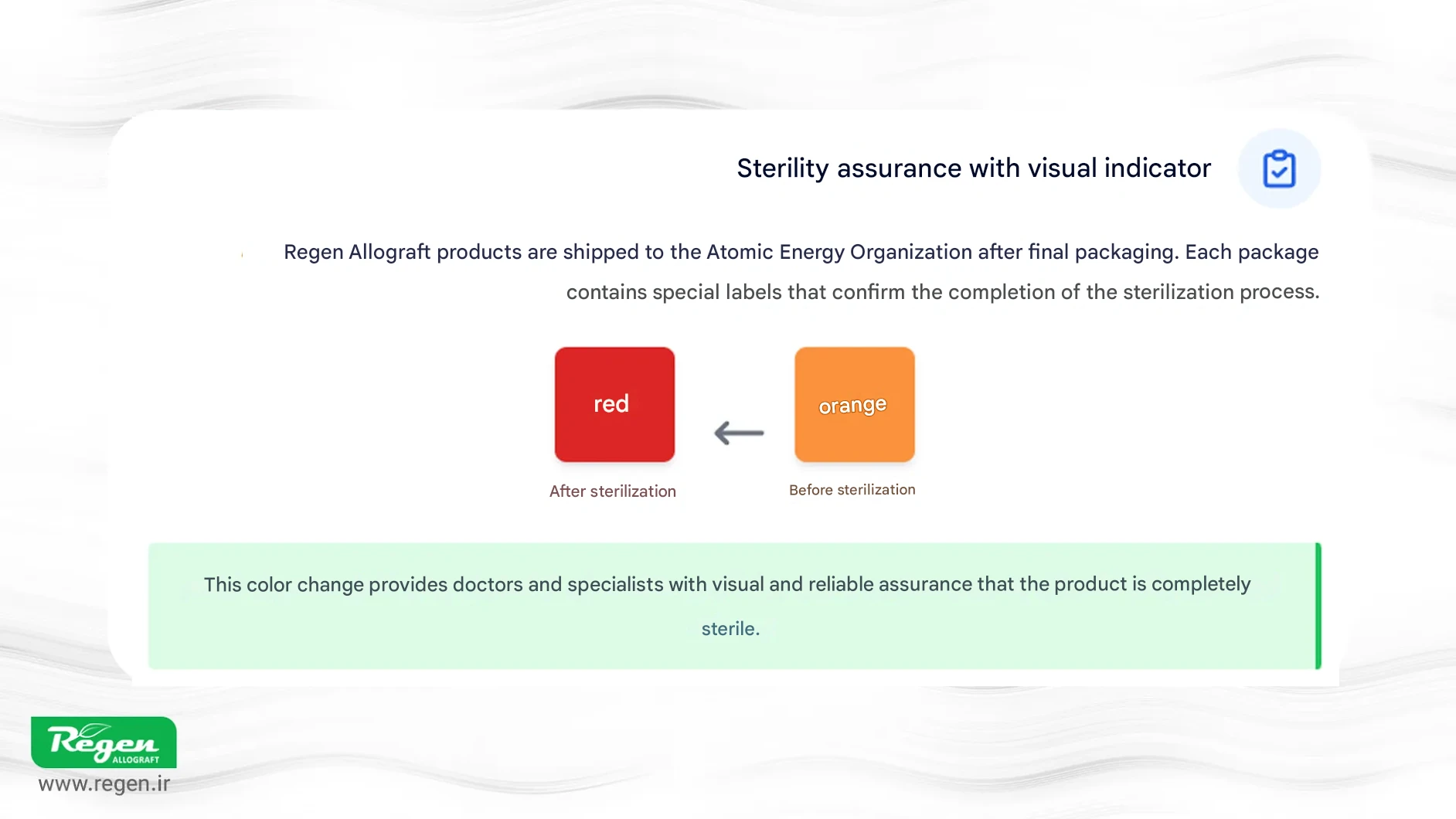
Regen Allograft (Iranian Tissue Products Company) sends all of its lyophilized products, in their final packaging, to the Atomic Energy Organization for gamma irradiation. Special indicator labels are incorporated in every packaging layer, which change color from orange to red once the sterilization cycle through gamma irradiation is fully completed. This feature serves as a reliable visual indicator, providing physicians and specialists with assurance of complete sterility. Such commitment to quality, efficacy, and safety makes these products a trusted choice for professionals in orthopedics, dentistry, maxillofacial surgery, and other medical fields where these grafts are utilized.

Sterilization Methods of Allografts and the Role of Gamma Irradiation
To date, various methods have been employed for the sterilization of transplantable tissues, as reported in published studies. The most notable methods include gamma irradiation, electron beam (accelerator) irradiation, ethylene oxide gas, moist heat treatment, chemical sterilization (e.g., peracetic acid), and antibiotic incubation. Each of these methods has its own advantages and limitations. For instance, ethylene oxide gas has good penetrative ability but leaves toxic residues (ethylene chlorohydrin), which can trigger inflammatory reactions. Chemical methods like peracetic acid can be effective but may compromise the biomechanical strength of certain tissues, such as ACL tendons.
Among these methods, gamma irradiation is widely preferred as it is a cold terminal sterilization technique performed after tissue packaging and leaves no harmful chemical residues. Gamma rays are highly penetrating ionizing radiation (typically sourced from cobalt-60 or cesium-137) that inactivate microbes and viruses by inducing DNA strand breaks.
Cryopreserved products, such as tendons and heart valves, are not exposed to gamma irradiation and are instead processed aseptically.
The residue-free nature of gamma irradiation is particularly important for medical materials, as it prevents unwanted reactions or impairment of the tissue’s regenerative response. Additionally, due to its high penetrative capacity, gamma irradiation can fully sterilize dense tissues, such as thick bones, whereas methods like moist heat or chemical sterilization may not penetrate sufficiently. For these reasons, many tissue banks worldwide adopt gamma irradiation (within a defined dose range) as the standard sterilization method for allograft bones, cartilage, skin, and other transplantable tissues.
However, the dose intensity and irradiation conditions can influence the quality of the tissue, and must therefore be carefully controlled.
Effects of Gamma Irradiation on the Mechanical Properties of Allografts
The protein structure of collagen is the primary determinant of the strength and flexibility of bone and connective tissues. Studies have shown that gamma irradiation can damage the mechanical properties of bone allografts by inducing degradation of collagen fibers and altering intra- and intermolecular cross-links. In other words, gamma rays generate free radicals in the tissue (through water radiolysis), leading to collagen degradation and abnormal cross-linking, which reduces the flexibility (plasticity) of the bone.
Effects of Gamma Irradiation on Bone Allografts
Bone, as one of the most commonly used allografts in orthopedics and dentistry, requires reliable sterilization more than any other tissue. Gamma irradiation, due to its high penetrative ability, can reduce the risk of disease transmission to near zero; however, this process may have implications for the mechanical properties of bone. Studies have shown that gamma radiation, depending on the absorbed dose, can decrease the Young’s modulus and bending strength of bone and alter its collagen structure. Nevertheless, when sterilization is performed within the optimal dose range (15–25 kGy), the reduction in strength is not significant enough to compromise the clinical utility of the bone. In practice, products such as bone powder, chips, and putty retain their therapeutic performance even after gamma irradiation and remain a safe and effective choice for bone tissue reconstruction
Effects on Allograft Collagen Membranes
Skin allografts, such as Soft Derm and Acellular Dermal Matrix, as well as other collagen membranes like Pericard and Fascia (one of the well-known types), play an important role in soft tissue repair—from healing extensive burn-related skin defects to providing collagen coverage in periodontal surgeries.
Collagen membranes, used as biological scaffolds in dentistry, implantology, and soft tissue reconstruction, are highly sensitive to sterilization processes. Gamma irradiation can alter the structure of collagen fibers and, at high doses, reduce the elasticity and flexibility of the membrane. However, within the optimal dose range (15–25 kGy), mechanical properties remain acceptable, and importantly, the biocompatibility of the membrane and its ability to induce reparative responses and support cell adhesion are preserved. For this reason, gamma irradiation is still recognized as a standard and reliable method for sterilizing collagen membranes and is widely used in tissue bank products.
Studies have shown that gamma irradiation can densify collagen structures in skin matrices and may even create microcavities in collagen fibers. Research on AlloDerm indicated that at low gamma doses (e.g., 2–5 kGy), there was a slight increase in tensile strength, likely due to increased cross-linking in collagen. However, with higher doses, tensile strength decreased. The elasticity or flexibility of the skin matrix is significantly reduced after irradiation; in simple terms, gamma-irradiated dermal tissue becomes stiffer and less stretchable.
Conclusion and Commitment to Safety Standards
Gamma irradiation, as a powerful tool, plays a pivotal role in ensuring the safety of bone allografts and collagen membranes. By effectively eliminating infectious agents, this method enables the widespread use of donated tissues in surgical procedures and, in many cases, reduces the need for autografts. Although high-dose gamma irradiation can somewhat affect the mechanical and biological properties of tissues, current knowledge allows these effects to be minimized through careful control of dose and irradiation conditions, ensuring that the clinical performance and functional properties of allografts are preserved.
Clinical experience over recent decades has demonstrated that gamma-sterilized bone allografts—including bone powders, chips, and structural pieces such as bone blocks and cubes—as well as membranes such as pericardium, fascia, and acellular dermis, achieve successful outcomes in tissue repair, with complication rates very low and comparable to traditional methods. Accordingly, gamma irradiation continues to be regarded as the gold standard for safety in the production of allograft products.

Finally, it is noteworthy that in Iran, knowledge-based companies also produce safe allografts in compliance with international standards. Regen Allograft, as a human allograft manufacturer in the country, processes its products—from various bone grafts (powder, membranes, chips, etc.) to dermis and cartilage—under the supervision of competent authorities and in accordance with AATB and Euro GTP protocols.
By obtaining quality certifications such as ISO 13485 and implementing a cleanroom system throughout all production stages, the company ensures that donated tissues, after rigorous screening, are packaged under fully sterile conditions. The final sterilization of all products is performed via gamma irradiation by the Atomic Energy Organization of Iran, providing the highest level of microbial safety assurance.
This commitment to quality, efficacy, and safety has established Regen Allograft products as a trusted choice for specialists in orthopedics, dentistry, maxillofacial surgery, and other related fields. Adhering to international safety and sterilization standards promises a bright future for tissue transplantation in Iran.
References
• Singh R, et al. Radiation sterilization of tissue allografts: A review. World J Radiol. 2016;8(4):355-369.
• Arjmand B, et al. Effect of gamma irradiation on osteoinduction of demineralized bone powder allograft. J Med Council I.R. Iran. 1386;25(1):9-16.
• Ku JK, et al. Effect of Gamma Irradiation on the Osteoinductivity of Demineralized Dentin Matrix. J Funct Biomater. 2022;13(1):14.
• Gouk SS, et al. Alterations of human acellular dermal matrix by gamma irradiation. J Biomed Mater Res B. 2008;84(1):205-217.
• Woo SJ, et al. Comparison of irradiated and non-irradiated acellular dermal matrices in breast reconstruction. Arch Plast Surg. 2021;48(1):54-61.
How useful was this post?
Click on a star to rate it!
Average rating 5 / 5. Vote count: 9
No votes so far! Be the first to rate this post.
Relevant Posts

Free consultation
Do you need counseling?
The professional and specialized team at Allograft is ready to assist you