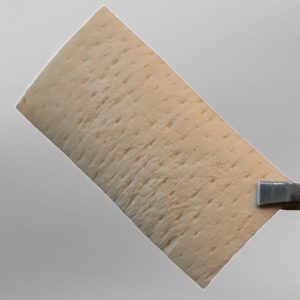
Fascia Lata
The product is processed from human Fascia Lata and is available as lyophilized, radiated products in various sizes. Due to its flexibility, adhesiveness, and biocompatibility, it can easily be used at the operation site and causes volume enhancement and mechanical strength.
Features & Indications
- Epithelial cell direction in different types of implant covers
- Anatomic space separation in various types of fractures and cleft palates
- Fixation and prevention from washing out during bone powder use
- GBR and GTR
- Gingival surgery in cases that primary closure was not achieved
- Functional, traumatic, and cosmetic (rhinoplasty) reconstructions
- Dermis thickness increase in cases with thin skin at the surgical site in facial and dorsal cosmetic surgeries
- Duraplasty, as an alternative for human dura mater (neurosurgery)
- Facial defect repair and soft tissue reconstruction (plastic surgery)
- As a sling in ptosis and blepharoplasty surgeries (ophthalmology)
- Bladder Neck Suspension in urologic surgeries
- Abdominal herniation repair (abdominal surgery)
- Rotator cuff, tendon, and ligament reconstruction (orthopaedics)
- Vascular surgery
|
Product Code |
Product Name |
Therapeutic Area |
|---|---|---|
|
5010022 |
Lyophilized Fascia Lata Membrane T<0.5 mm S:10×15 mm |
Dental |
|
5010023 |
Lyophilized Fascia Lata Membrane T<0.5 mm S:15×20 mm |
Dental |
|
5010024 |
Lyophilized Fascia Lata Membrane T<0.5 mm S:20×30 mm |
Dental |
|
5010025 |
Lyophilized Fascia Lata Membrane T<0.5 mm S:20×40 mm |
Dental |
|
5010026 |
Lyophilized Fascia Lata Membrane T<0.5 mm S:25×50 mm |
ENT, Plastic Surgery |
|
5010027 |
Lyophilized Fascia Lata Membrane T<0.5 mm S:45×85 mm |
ENT, Plastic Surgery |
|
5010028 |
Lyophilized Fascia Lata Membrane T<0.5 mm S:85×85 mm |
ENT, Plastic Surgery |
|
5010029 |
Lyophilized Fascia Lata Membrane T:0.5-1 mm S:10×15 mm |
Dental |
|
5010030 |
Lyophilized Fascia Lata Membrane T:0.5-1 mm S:15×20 mm |
Dental |
|
5010031 |
Lyophilized Fascia Lata Membrane T:0.5-1 mm S:20×30 mm |
Dental |
|
5010032 |
Lyophilized Fascia Lata Membrane T:0.5-1 mm S:20×40 mm |
Dental |
|
5010033 |
Lyophilized Fascia Lata Membrane T:0.5-1 mm S:25×50 mm |
ENT, Plastic Surgery |
|
5010034 |
Lyophilized Fascia Lata Membrane T:0.5-1 mm S:45×85 mm |
ENT, Plastic Surgery |
|
5010035 |
Lyophilized Fascia Lata Membrane T:0.5-1 mm S:85×85 mm |
ENT, Plastic Surgery |
|
5010036 |
Lyophilized Fascia Lata Membrane T>1 mm S:25×50 mm |
ENT, Plastic Surgery |
|
5010037 |
Lyophilized Fascia Lata Membrane T>1 mm S:45×85 mm |
ENT, Plastic Surgery |
|
5010038 |
Lyophilized Fascia Lata Membrane T>1 mm S:85×85 mm |
ENT, Plastic Surgery |
Related products
Acellular Dermis Membrane
Cartilage & Septum
DBM Putty
Demineralized bone matrix (DBM) putty is a highly specialized bone graft material that has emerged as a valuable tool in the surgical management of bone defects. Derived from human or animal bone that has been processed to remove its mineral components, DBM putty presents a soft, malleable scaffold richly endowed with osteoconductive and osteoinductive properties. These properties enable DBM putty to serve as a platform for bone cell attachment, proliferation, and differentiation, fostering the regeneration of new bone tissue.
DBM putty offers a range of advantages over traditional autografts (bone grafts harvested from the patient's own body) and allografts (bone grafts sourced from cadavers). Its osteoconductive nature allows it to seamlessly integrate with the surrounding bone, while its osteoinductive capacity stimulates the production of bone-forming proteins, accelerating the healing process.
The biocompatible carrier in which DBM putty is incorporated plays a crucial role in its moldable and easy-to-form nature. This carrier, typically a combination of collagen and alginate, provides the putty with its desired consistency, allowing surgeons to precisely shape and contour it to fill bone voids of various sizes and configurations. The carrier also contributes to the overall bioactivity of DBM putty, enhancing its ability to promote bone regeneration.
Particulated Bone
Particulated bone allografts, derived from human bone tissue, are biomaterials used to fill bone defects and promote bone regeneration. They come in various particle sizes, each offering unique properties and applications.
Powdered allografts, the smallest particles, possess high integration capabilities and are suitable for small defects. Granule and crushed allografts are larger in size, serving as a foundation for bone regeneration and can be used as fillers or for augmentation.
Chip allografts, the largest among particulated bone grafts, provide a bone scaffold, making them ideal for larger defects or augmentation.