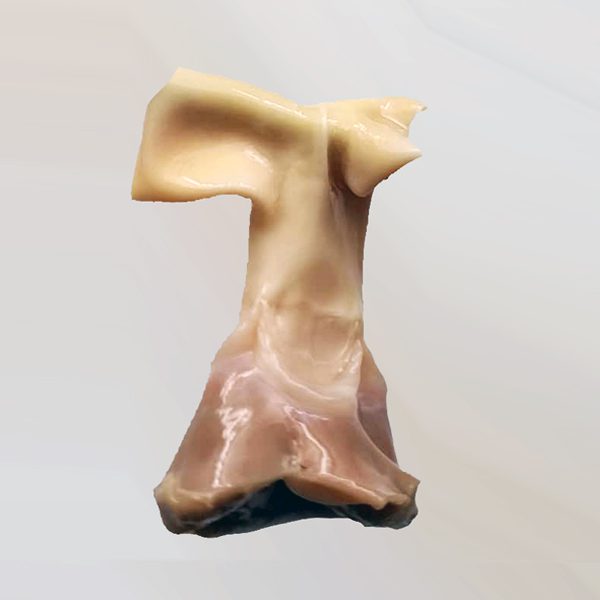
Heart Valve
Choose File
The heart valve allograft is produced and processed by the Iranian Knowledge-Based Company of Tissue Products from donated human heart valve tissue. During the processing of this product, an antibiotic cocktail is employed. Aortic and pulmonary heart valve allografts are utilized in cardiovascular surgeries and are indicated for the repair of congenital heart diseases and valvular insufficiencies. In addition, they are applied for the replacement or reconstruction of damaged or dysfunctional heart valves.
Product Description
Benefits
- Biocompatible with surrounding blood cells and able to restore the patient’s normal blood flow
- The avoidance of lifelong warfarin therapy is required for preventing the emergence of blood clots
- Negligible risk of disease transmission/infection regarding the performed restrict quality assurance
- Low rejection or mortality rate in long-term due to its optimum capability of mimicking the native tissue function compared to its alternatives such as mechanical heart valves
- Availability in various sizes to best fit the patient’s anatomy
- Effective orifice area closely approximates the native valve
- Cost-Effectiveness in comparison to its efficacy
Clinical Application
Valves are used in cardiovascular surgery for the aim of repairing congenital heart diseases and aortic valvular insufficiency. Moreover, it is intended for the replacement or reconstruction of diseased, damaged, malformed, and malfunctioning prosthetic heart valves.
Storage Condition
The product is packaged in nylon film in two layers and finally in an aluminum envelope. The package should be inspected before use to ensure that it is not damaged. This product is stored in liquid nitrogen vapor phase conditions (-150° to -187° Celsius) for up to 5 years. Also, it could be stored at -80° Celsius for up to 6 months.
Product Code | Product Name | Therapeutic Area |
|---|---|---|
5060020 | Cryopreserved Heart valve Aortic L=40-65 D=9-31 mm | Cardiovascular |
5060021 | Cryopreserved Heart valve Pulmonary L=40-65 D=9-31 mm | Cardiovascular |
Contact us
Get expert advice
Contact our experts for free guidance and advice, or read our About Us page to learn more about us.
Related content
Some of our products
The acellular dermis membrane collagen membrane, contains basement membrane on one of its surfaces. It can provide a dense and impenetrable layer against microorganism and pathogen penetration into deeper layers.
So the membrane surface can stay exposed in high-perfused tissue of the oral cavity. The product origin is the collagen membrane (skin dermal layer), which consists of strong collagen type I and III fibers that strengthen the product, and also elastic fibers causing flexibility. This collagen membrane results in product’s unique features.
Freeze-dried bone allograft as Bone grafting such as Cube, Matchstick and Block forms has revolutionized orthopedic and maxillofacial surgeries, offering distinct advantages due to their unique compositions. Cube and Matchstick configurations consist solely of cancellous bone, while block formations comprise a core of cancellous bone enveloped by a cortical surface. These allograft are meticulously processed to decellularization and ensure sterilization. The resulting grafts provide essential structural support and seamlessly integrate with the recipient’s bone, promoting the formation of new bone.
Cube and block bone allografts effectively address osseous deficiencies caused by various conditions, from post-traumatic injuries to degenerative diseases and reconstructive procedures. In dental implantology, they serve as a reliable scaffold for augmenting deficient mandible and maxilla structures and facilitating successful implant placement. Moreover, in reconstruction surgeries, these grafts significantly contribute to restoring maxillofacial and skeletal, enabling patients to regain both physical form and function.
Ongoing refinement of bone grafting techniques, along with continued research and innovation, will further enhance the applications and outcomes of cube and block bone allografts, elevating the standard of care in orthopedic and maxillofacial surgeries.