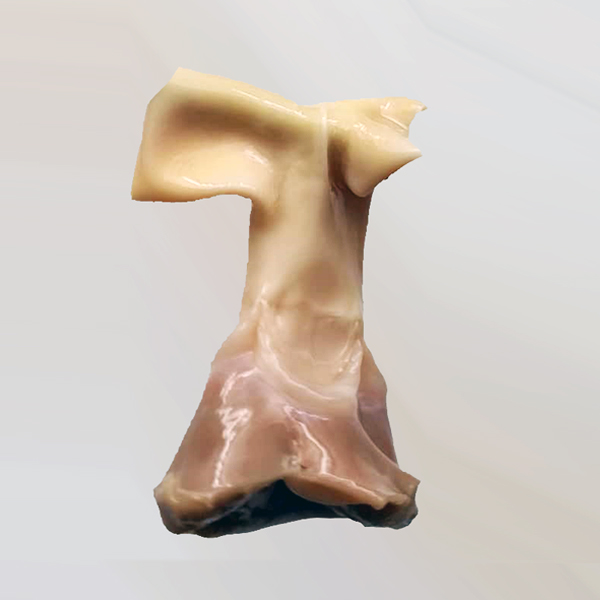
Heart Valve
The allograft heart valve product is derived from a cadaveric donated heart 48h after cardiac arrest. Prior to retrieving the whole heart from a mortuary or dedicated donation suite, the donor screening in case of age limitation (1 to 55 years old), medical history, and physical examination is performed. Then, the valve is carefully dissected from the heart in a licensed pharmaceutical grade cleanroom (GMP classification B) to remove excess myocardium, fat, and connective tissue. At this stage, the valve is treated with an antibiotic cocktail to reduce the bioburden. It should also be noted that in order to assure the product safety, several quality control tests such as microbiologic & serologic assays are carried out; each sample is tested for aerobic and anaerobic bacteria, fungi & HIV antibodies type 1, 2 (anti- HIV1&2), antibody to human T-lymphotropic virus type 1,2 (HTLV Ab1,2), Hepatitis B core antibody (HBcAb), Hepatitis C virus antibody (HCVAb), Hepatitis B surface Antigen (HBsAg), and RPR for syphilis.