Iranian Tissue Products Company
Iranian Tissue Products Company was founded in 2011 on the initiative of a group of faculty members and staff of Tehran University of Medical Sciences. The company’s activities initially began as a private company in the university’s Medical Equipment Development Center… Read more
Knowledge-Based Company
Established in 2011 by the faculty members of Tehran University of Medical Sciences
Indigenous Technology & Innovation
Expanding production by relying on up-to-date knowledge and localizing new technologies
Global Quality Assurance
Compliant with ISO 13485:2016 standards and AATB protocols
Wide Medical Applications
Providing solutions in cardiac, orthopedic, dental and reconstructive surgeries
Valid Certificates
Knowledge-Based Company
Over 20 Specialized Products
Over 100 Medical Facilities
Product Classification by Tissue Type

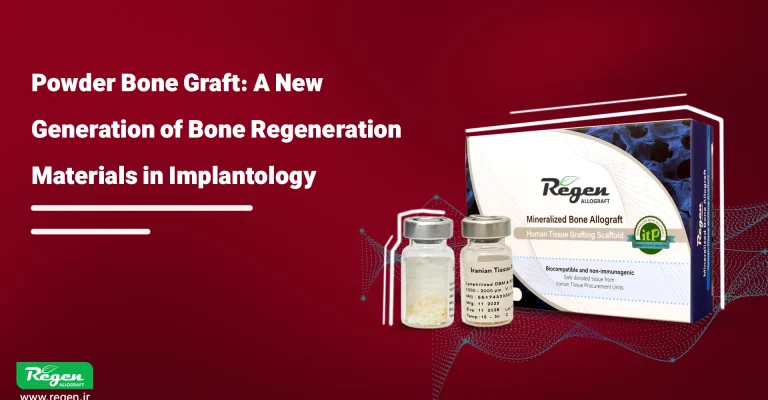
bone
skin
Fascial Lata

Cartilage

Combinational

Heart Valves

Pericardium
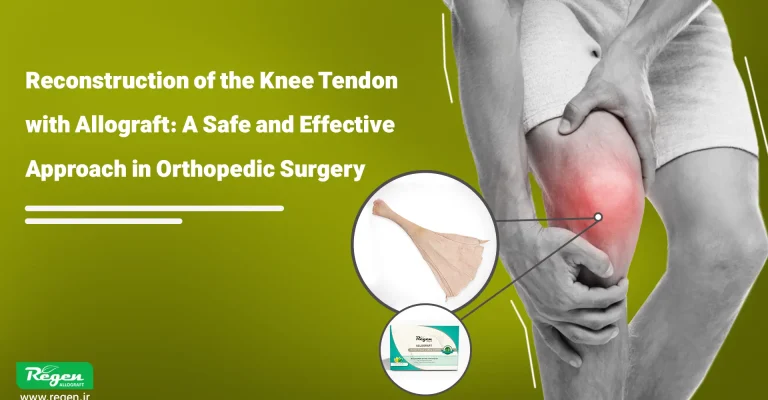
Tendons

Combinatorial

Heart Valve

Pericardium

Tendon
Our Specialized Sales Network
We are always expanding our sales network and inviting experts to join us
Iranian Tissue Products Company at a Glance
year of Experience
Specialized products
50+
Active Representatives
Scientific articles
year of Experience
Specialized products
Active Representatives
+50